A round up of recent industry news on the topics of Big Data and Enterprise Data Management
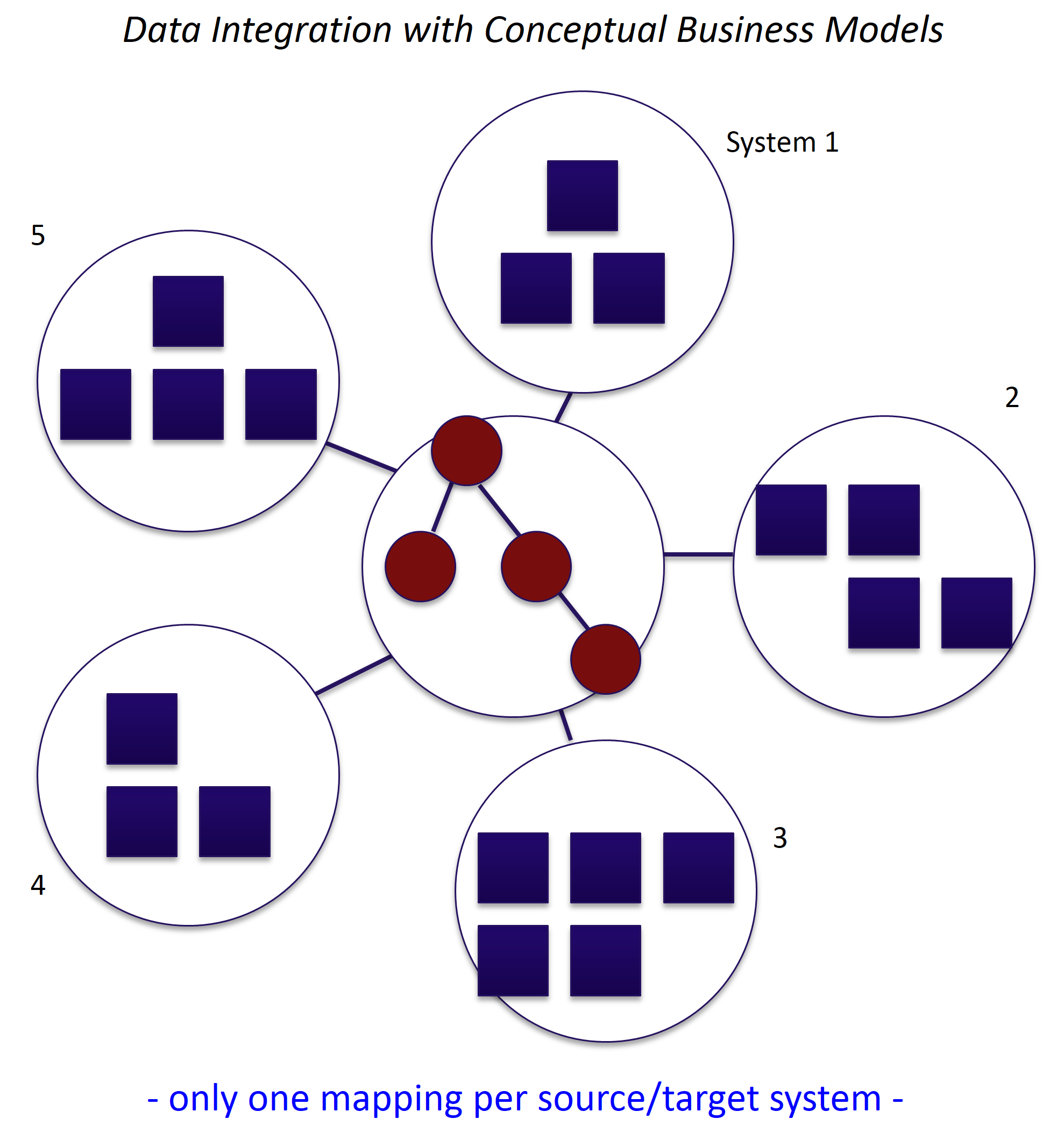
With CDISC Adoption by FDA, Managing Clinical Trial Metadata with Semantic Technology Has Emerged Front and Center!
As FDA outlines, “the submission of standardized study data enhances a reviewer’s ability to more fully understand and characterize the efficacy and safety of a medical product,” and adopted CDISC...