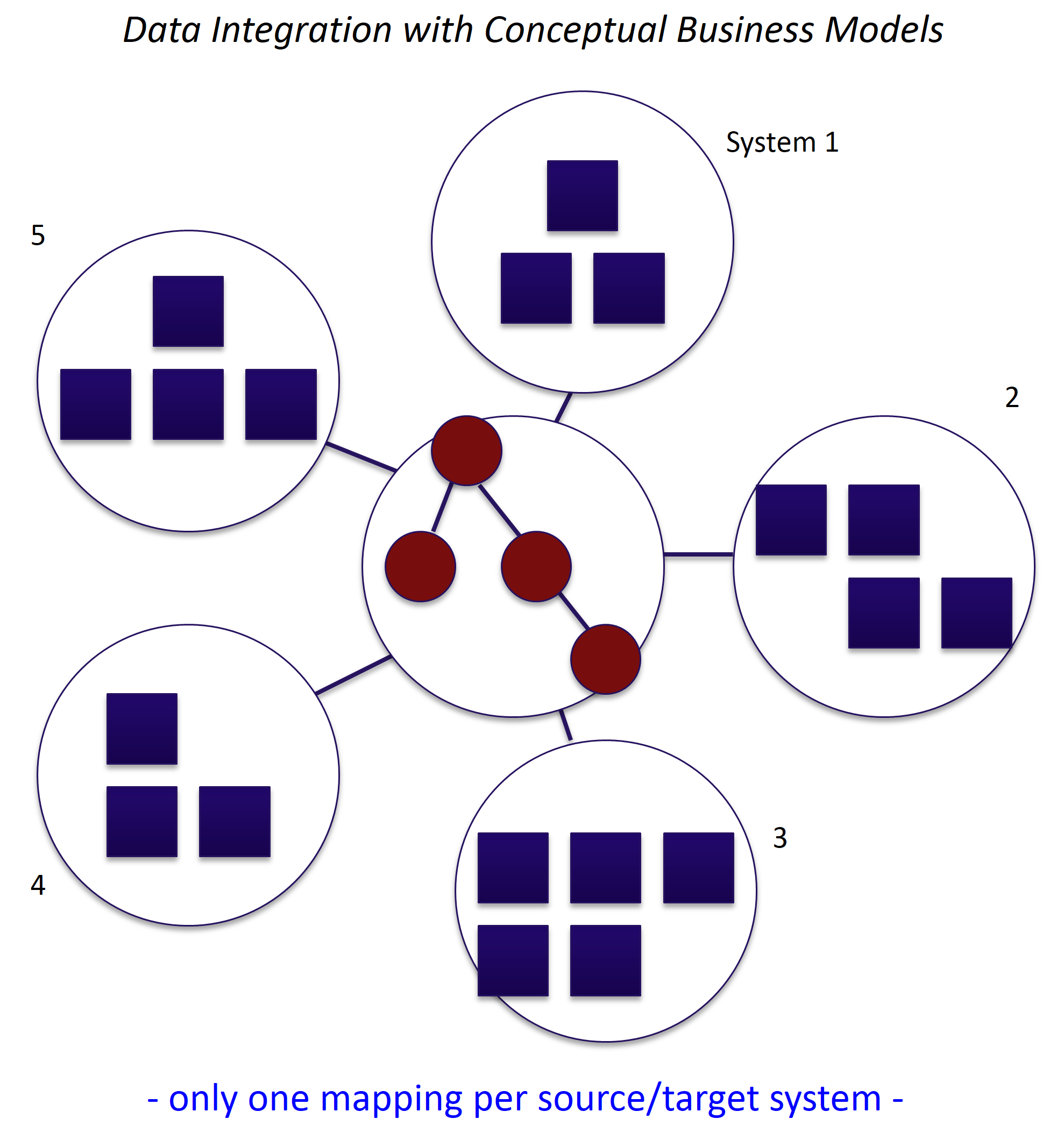
Driving business value from your data often requires integration across many sources. These integration projects can be time consuming, expensive and difficult to manage. Any short cuts can...
With CDISC Adoption by FDA, Managing Clinical Trial Metadata with Semantic Technology Has Emerged Front and Center!
As FDA outlines, “the submission of standardized study data enhances a reviewer’s ability to more fully understand and characterize the efficacy and safety of a medical product,” and adopted CDISC...