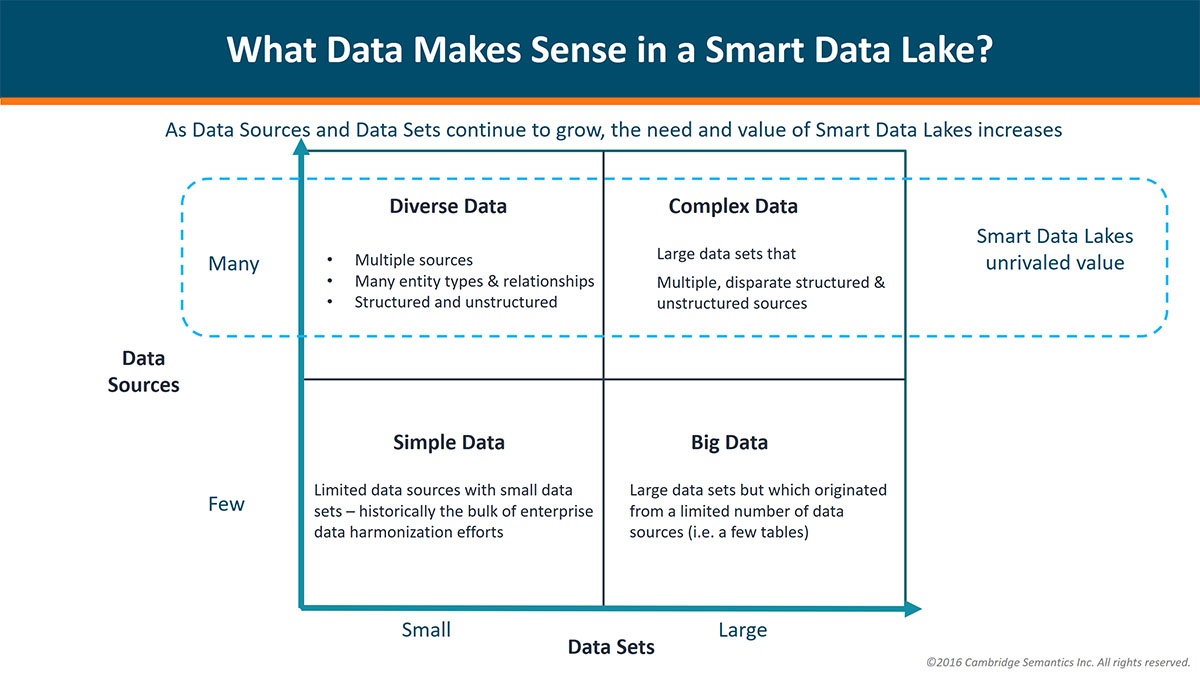
A biomedical professional is certain to have come across some variant of the headlines mentioned in the visual above. Combinations of trending buzzwords in technology and healthcare form half of my...
With CDISC Adoption by FDA, Managing Clinical Trial Metadata with Semantic Technology Has Emerged Front and Center!
As FDA outlines, “the submission of standardized study data enhances a reviewer’s ability to more fully understand and characterize the efficacy and safety of a medical product,” and adopted CDISC...